Transforming Dirty Water into Clean Energy
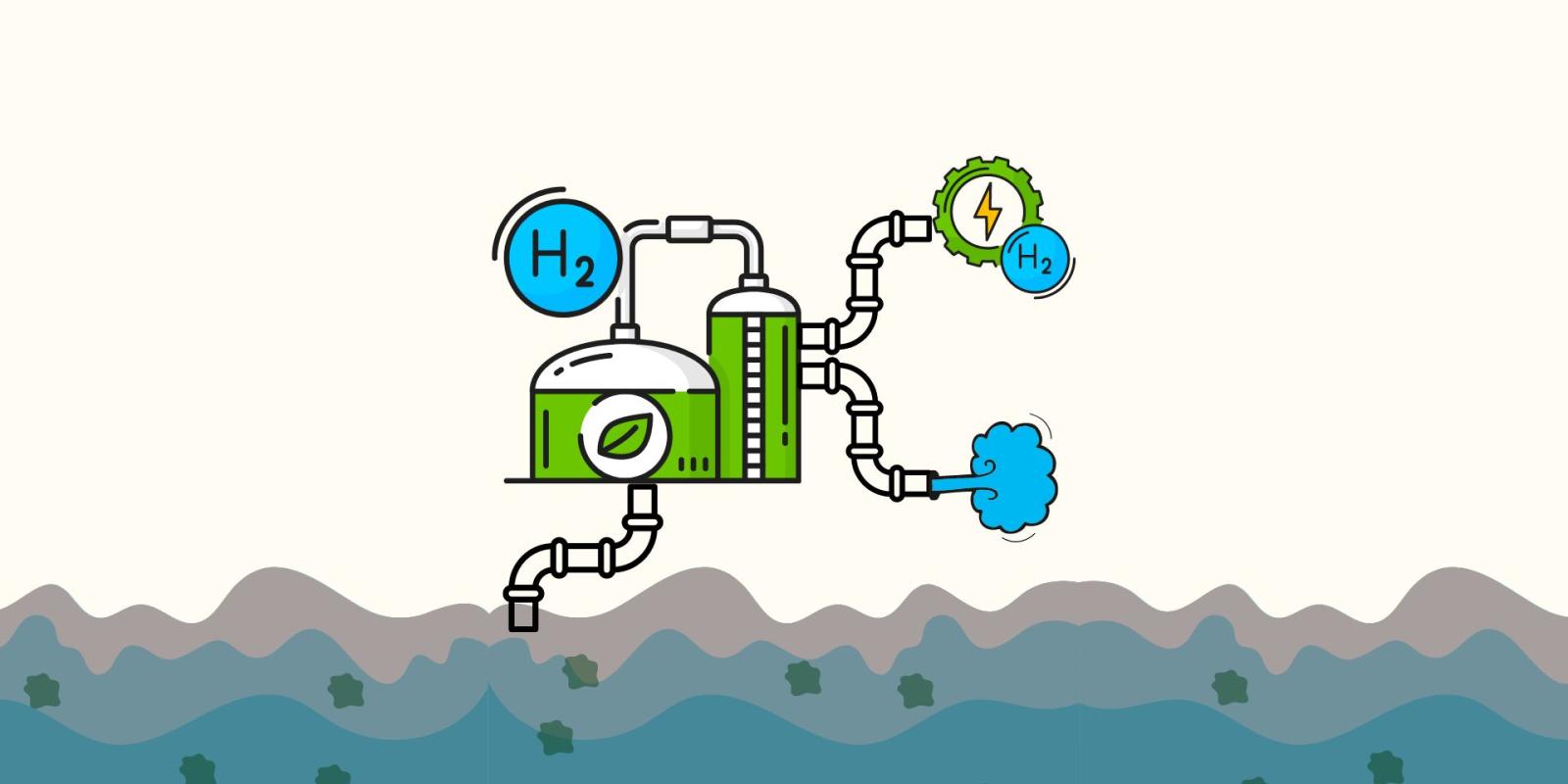
A bolt of energy rattles the bonds of a water molecule: H2O. The links between the molecule begin to snap as the oxygen and hydrogen particles are pulled to separate compartments. The hydrogen molecules are collected and pressurized until their gaseous form transforms into a liquid, the process guiding these powerful particles to their new life: green energy. This process, called electrolysis, has existed for several years; now the goal is to expand its application by producing green hydrogen and clean water simultaneously.
 Ehab El Sawy, assistant professor in the Department of Chemistry, recently received funding from Egypt’s Academy of Scientific Research and Technology to pursue this endeavor.
Ehab El Sawy, assistant professor in the Department of Chemistry, recently received funding from Egypt’s Academy of Scientific Research and Technology to pursue this endeavor.
“Traditional forms of green hydrogen production utilize clean water in their process. This is more expensive and inefficient; clean water should be prioritized for necessities like drinking,” El Sawy explains. “Our proposal is to utilize wastewater and salt water to produce the hydrogen energy in a way that generates clean water as a byproduct.”
This solution is a two-for-one deal. A small village with a contaminated lake could use this new technology both as an energy supplier and as a way to make its water safe for drinking.
But why green hydrogen?
“It has local and global benefits,” El Sawy says. “Right now, most of our energy comes from fossil fuels that produce toxic gasses like carbon dioxide, sulfur oxides and nitrogen oxides — all of which are an environmental disaster. Green hydrogen requires energy to create, so making it ‘green’ means ensuring the source of energy for the electrolysis is renewable, like wind, solar, geothermal, or tidal sources.”
Green hydrogen has become a popular alternative to fossil fuels internationally over the past two decades, with buses, trains and cars running on this climate-friendly alternative from the United States to Japan. In addition to its liquid form, which can be pumped like traditional gas, green hydrogen can also be put into fuel cells, similar to batteries. In the future, these fuel cells may be able to power houses and buildings as a replacement for power grids.
This climate-friendly energy is perfect for Egypt. “Here, we don’t have an abundance of fossil fuels like the Gulf region or lithium for lithium-based batteries like Australia,” El Sawy explains. “What we do have is plenty of sun and salt water.”
This project is interdisciplinary: El Sawy along with Nageh Allam, professor in the Department of Physics, use their expertise in electrochemistry and materials design to refine the compartments that produce the hydrogen through electrolysis so they can function at max efficiency. Meanwhile other AUC faculty, like Ahmed El-Gendy, professor and director of the environmental engineering graduate program, and Anwar Abd ElNaser, assistant professor in the Department of Chemistry, study the water treatment and desalination system. El-Gendy and Abd ElNaser monitor bacterial growth on electrodes, desalination efficiency and the physics of the molecular movements, in addition to exploring the benefits of this new technology on public health.
AUC students are also involved in the process. Undergraduate and graduate research assistants help develop the materials in the lab throughout the school year and summer. Recent PhD graduates are also funded in the lab, offering them the opportunity to conduct research while attending international conferences and workshops.
“It has been a privilege to work with such a collaborative team,” El Sawy says. “Our project is still in the initial stages, but with more funding and research, I believe we can turn this design into a reality.
